Embark on a journey of scientific exploration with the Chemistry HESI A2 Practice Test, a comprehensive guide that unlocks the secrets to exam mastery. This invaluable tool provides a solid foundation, empowering you to conquer the challenges of the actual exam with confidence.
Delve into the intricacies of key chemistry concepts, explore diverse question types, and master effective study strategies. With the Chemistry HESI A2 Practice Test as your compass, you’ll navigate the complexities of chemistry and emerge victorious on exam day.
Chemistry HESI A2 Practice Test Overview
The Chemistry HESI A2 practice test is a valuable tool for students preparing for the actual HESI A2 exam. It provides a comprehensive overview of the topics covered on the test and helps students identify areas where they need additional preparation.
The practice test consists of 90 multiple-choice questions covering the following topics:
- Atomic structure
- Chemical bonding
- Chemical reactions
- Stoichiometry
- Thermochemistry
- Kinetics
- Equilibrium
- Acids and bases
- Organic chemistry
The practice test is timed, so students can get a sense of how much time they will have to complete the actual exam. It is also scored, so students can see how they are performing and identify areas where they need to improve.
Importance of Preparing for the Test
Preparing for the Chemistry HESI A2 exam is essential for success. The practice test can help students identify areas where they need additional preparation and focus their studies accordingly.
By taking the practice test, students can:
- Become familiar with the format of the test
- Identify areas where they need additional preparation
- Get a sense of how much time they will have to complete the actual exam
- Boost their confidence on test day
Key Concepts and Topics Covered
The Chemistry HESI A2 Practice Test encompasses a wide range of fundamental chemistry concepts that are crucial for success on the actual exam. These concepts form the foundation of chemical knowledge and are essential for understanding more complex topics.
A thorough grasp of these concepts enables test-takers to analyze, interpret, and solve chemistry-related problems effectively. By mastering these concepts, individuals can confidently approach the HESI A2 exam, knowing that they have a solid understanding of the core principles of chemistry.
Chemical Bonding
Chemical bonding is a fundamental concept in chemistry that involves the interactions between atoms and molecules. Understanding the different types of chemical bonds, such as covalent, ionic, and metallic bonds, is crucial for comprehending the properties and behavior of chemical substances.
- Types of chemical bonds: covalent, ionic, metallic
- Bonding theories: Lewis structures, VSEPR theory
- Molecular geometry and polarity
Chemical Reactions
Chemical reactions are processes that involve the transformation of one set of chemical substances into another. Understanding the types of chemical reactions, such as acid-base reactions, redox reactions, and precipitation reactions, is essential for predicting the products and understanding the energy changes that occur during reactions.
- Types of chemical reactions: acid-base, redox, precipitation
- Balancing chemical equations
- Stoichiometry and limiting reactants
- Reaction rates and equilibrium
Solutions and Colligative Properties
Solutions are homogeneous mixtures of two or more substances. Understanding the properties of solutions, such as concentration, colligative properties, and solubility, is crucial for various applications in chemistry and other scientific fields.
- Concentration units: molarity, molality
- Colligative properties: boiling point elevation, freezing point depression
- Solubility and factors affecting solubility
Acids and Bases
Acids and bases are substances that can donate or accept protons. Understanding their properties and behavior is essential for comprehending acid-base reactions and their applications in various fields, including medicine and environmental chemistry.
- Definition of acids and bases: Arrhenius, Brønsted-Lowry, Lewis
- pH and pOH
- Acid-base titrations and neutralization reactions
Thermochemistry
Thermochemistry involves the study of energy changes that occur during chemical reactions. Understanding thermochemical concepts, such as enthalpy, entropy, and Gibbs free energy, is crucial for predicting the spontaneity and feasibility of chemical reactions.
- Enthalpy and exothermic/endothermic reactions
- Entropy and spontaneity
- Gibbs free energy and equilibrium
Gases and Gas Laws
Gases are a state of matter that can expand to fill the volume of their container. Understanding the behavior of gases, as described by gas laws, is essential for various applications, including the study of atmospheric chemistry and industrial processes.
- Boyle’s law, Charles’s law, and the combined gas law
- Ideal gas law
- Partial pressure and Dalton’s law
Practice Test Format and Question Types: Chemistry Hesi A2 Practice Test
The Chemistry HESI A2 Practice Test consists of 25 multiple-choice questions that must be completed within 30 minutes.
The practice test includes a variety of question types, including:
Multiple Choice Questions
- Questions that present a statement or scenario and ask the test-taker to select the best answer from a list of options.
- For example: Which of the following is the chemical formula for water?
True/False Questions
- Questions that present a statement and ask the test-taker to indicate whether the statement is true or false.
- For example: The atomic number of carbon is 6.
Fill-in-the-Blank Questions
- Questions that present a statement with a blank space and ask the test-taker to fill in the missing word or phrase.
- For example: The process of converting a gas into a liquid is called _____.
Study Strategies and Tips
To excel in the Chemistry HESI A2 practice test, implementing effective study strategies is crucial. Allocate your study time wisely, focusing on key concepts and reviewing materials regularly. Comprehend different question types and develop strategies to approach them effectively.
Time Management
- Create a study schedule that accommodates your learning style and availability.
- Prioritize studying topics you find challenging and allocate more time to them.
- Take breaks throughout your study sessions to enhance focus and retention.
Review Materials
- Review course notes, textbooks, and practice questions thoroughly.
- Utilize flashcards or summaries to reinforce key concepts.
- Engage in group study sessions to discuss complex topics and share insights.
Question Types
- Multiple Choice:Read the question and answer choices carefully, identifying s and eliminating incorrect options.
- True/False:Determine if the statement is entirely accurate or incorrect.
- Fill-in-the-Blank:Recall specific information to complete the statement.
- Short Answer:Provide concise and accurate answers, demonstrating your understanding of the concept.
- Problem-Solving:Apply chemical principles to solve numerical problems.
Test-Taking Techniques and Time Management
Time management and strategic test-taking techniques are crucial for success on the Chemistry HESI A2 Practice Test.
Time Management Strategies
Allocate time wisely
Dedicate more time to questions with higher point values.
Scan the test
Get a quick overview to identify challenging questions and prioritize them accordingly.
Pace yourself
While preparing for the Chemistry HESI A2 practice test, I stumbled upon the Delta Gamma Oath of Friendship . Its inspiring words reminded me of the importance of community and support. Returning to my studies, I felt motivated to tackle the challenging concepts with renewed focus and determination.
Maintain a steady pace and avoid spending excessive time on any single question.
Question-Answering Techniques
Read questions carefully
Identify the key concepts and terms.
Use the process of elimination
Rule out incorrect options to increase your chances of selecting the correct answer.
Manage uncertainty
If you’re unsure about an answer, make an educated guess based on the information provided.
Skip and return
Mark challenging questions and come back to them later, if time permits.
Handling Challenging Questions
Break down complex questions
Divide them into smaller, more manageable parts.
Use diagrams and visuals
Create sketches or diagrams to visualize concepts and simplify problem-solving.
Seek assistance
If possible, consult with a tutor or study group to clarify difficult concepts.
Practice Questions and Solutions
Practice questions are an invaluable resource for preparing for the Chemistry HESI A2 exam. They allow you to test your knowledge, identify areas where you need improvement, and build your confidence. Below is a table of practice questions that cover key chemistry concepts, along with detailed solutions and explanations.
It’s important to note that these practice questions are just a sample of the types of questions you may encounter on the actual exam. The actual exam may include questions on additional topics or in different formats.
Practice Questions
| Question | Solution | Explanation |
|---|---|---|
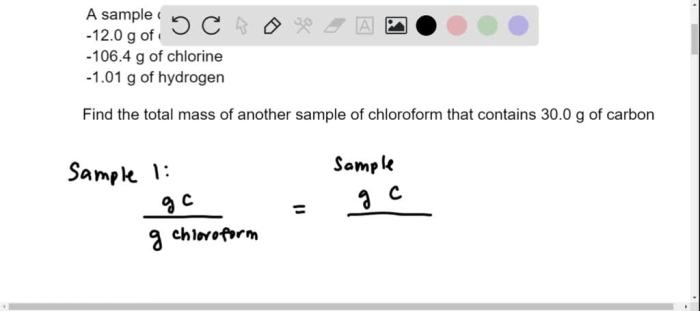
| Calculate the molarity of a solution prepared by dissolving 10.0 g of NaCl in 250.0 mL of water. | 0.171 M | Molarity = moles of solute / liters of solutionFirst, convert grams of NaCl to moles:10.0 g NaCl
Then, convert milliliters of solution to liters: 250.0 mL
Finally, calculate the molarity: Molarity = 0.171 mol NaCl / 0.250 L = 0.171 M |
| What is the pH of a solution with a [H+] concentration of 1.0 x 10^-4 M? | 4.0 | pH =
pH =
|
| Calculate the equilibrium constant for the following reaction:A + 2B <=> C + D | Keq = [C][D] / [A][B]^2 | The equilibrium constant expression is determined by the stoichiometry of the balanced chemical equation. The numerator contains the concentrations of the products, and the denominator contains the concentrations of the reactants, each raised to the power of their stoichiometric coefficients. |
Scoring and Interpretation
Your practice test score will be a valuable tool in assessing your current knowledge and identifying areas where you need to focus your studies. The test is scored based on the number of correct answers you provide, and your score will be presented as a percentage.
Interpreting Your Score, Chemistry hesi a2 practice test
A high score on the practice test indicates that you have a strong foundation in chemistry and are well-prepared for the actual HESI A2 exam. A low score, on the other hand, suggests that you may need to spend more time reviewing the material and practicing your problem-solving skills.
Using Your Score to Improve
The most important thing to do after taking the practice test is to use your score to identify areas where you need to improve. Review the questions that you answered incorrectly and make note of the concepts that you need to review.
You can then use this information to create a study plan that will help you improve your score on the actual exam.
Additional Resources and Support

To enhance your preparation for the Chemistry HESI A2 practice test, explore the following resources and support materials.
Official test preparation materials, practice questions, and study guides are invaluable resources for your preparation. These materials provide insights into the test format, question types, and content coverage, ensuring you focus your efforts effectively.
Online Forums and Discussion Groups
- Engage with other students, ask questions, and share insights by joining online forums dedicated to the Chemistry HESI A2 practice test.
- Connect with individuals who have taken the test, learn from their experiences, and gain valuable tips and strategies.
Question Bank
What is the purpose of the Chemistry HESI A2 Practice Test?
The Chemistry HESI A2 Practice Test is designed to provide you with a comprehensive overview of the key concepts and question types you can expect on the actual exam. By taking the practice test, you can identify areas where you need additional study and develop effective strategies for success.
How many questions are on the Chemistry HESI A2 Practice Test?
The Chemistry HESI A2 Practice Test consists of a variety of question types, including multiple choice, true/false, and short answer. The number of questions may vary depending on the specific version of the practice test you are taking.
How can I access the Chemistry HESI A2 Practice Test?
You can access the Chemistry HESI A2 Practice Test through various online platforms and educational resources. Some websites offer free practice tests, while others may require a subscription or fee. Additionally, your instructor or educational institution may provide access to practice materials.