A sample of chloroform is found to contain 12.0, a discovery that has sparked both scientific curiosity and concern. This intriguing compound, with its unique properties and multifaceted applications, warrants a thorough exploration. From its molecular structure and physical characteristics to its potential hazards and regulatory implications, this article delves into the captivating world of chloroform, unraveling its complexities and shedding light on its significance.
Chloroform, a colorless liquid with a distinctive pungent odor, has played a pivotal role in various industries and medical practices throughout history. Its versatility, however, is tempered by its inherent toxicity, necessitating careful handling and stringent safety measures. Understanding the intricate balance between chloroform’s benefits and risks is crucial for harnessing its potential while safeguarding human health and the environment.
Chemical Properties of Chloroform
Chloroform is a colorless, non-flammable liquid with a sweet, ethereal odor. It is a volatile compound that evaporates easily at room temperature. Chloroform is a halogenated hydrocarbon, meaning that it contains chlorine atoms bonded to a carbon atom. The molecular structure of chloroform is CHCl3, which means that it has one carbon atom, three hydrogen atoms, and three chlorine atoms.The
physical properties of chloroform include a boiling point of 61.2 °C, a melting point of63.5 °C, and a density of 1.48 g/mL. Chloroform is a relatively stable compound, but it can be decomposed by heat, light, or strong acids or bases.Chloroform
is a reactive compound that can undergo a variety of chemical reactions. It can react with other halogens to form carbon tetrachloride, chloroformate esters, and other compounds. Chloroform can also react with alcohols to form alkyl chlorides, and it can react with alkenes to form addition products.
Toxicity of Chloroform
Chloroform is a toxic compound that can cause a variety of adverse health effects. Exposure to chloroform can occur through inhalation, ingestion, or skin contact. The most common route of exposure is through inhalation of chloroform vapors.The toxic effects of chloroform include:* Central nervous system depression
- Respiratory depression
- Cardiac arrhythmias
- Liver damage
- Kidney damage
- Cancer
The symptoms of chloroform poisoning include:* Headache
- Dizziness
- Nausea
- Vomiting
- Confusion
- Loss of consciousness
- Respiratory depression
- Cardiac arrhythmias
Treatment for chloroform poisoning includes:* Removal from the source of exposure
- Supportive care
- Administration of activated charcoal
- Intravenous fluids
- Hemodialysis
Uses of Chloroform
Chloroform was once widely used as an anesthetic, but its use has declined in recent years due to the development of safer alternatives. Chloroform is still used in some countries as a solvent for oils, fats, and waxes. It is also used in the production of dyes, pharmaceuticals, and other chemicals.The
advantages of using chloroform include:* It is a powerful anesthetic
- It is relatively inexpensive
- It is easy to administer
The disadvantages of using chloroform include:* It is toxic
- It can cause liver damage
- It can cause kidney damage
- It can cause cancer
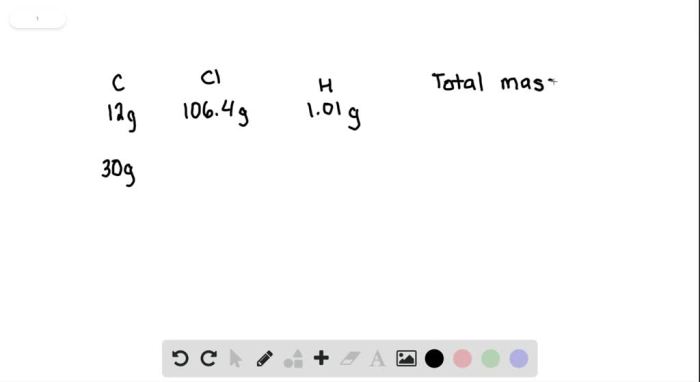
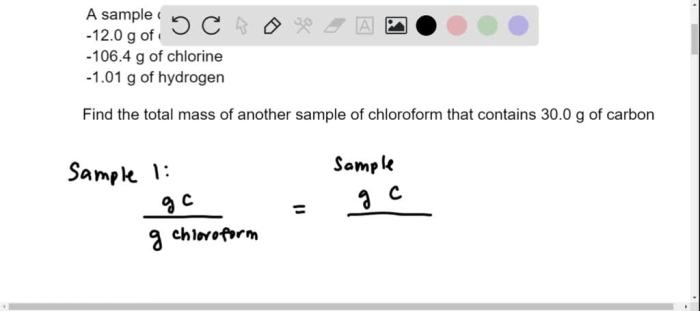
Detection and Measurement of Chloroform: A Sample Of Chloroform Is Found To Contain 12.0

Chloroform can be detected and measured in the environment using a variety of methods. These methods include:* Gas chromatography
- Mass spectrometry
- Infrared spectroscopy
- Ultraviolet spectroscopy
These methods are all based on the principle of detecting the presence of chloroform by measuring its physical or chemical properties.The detection and measurement of chloroform in the environment is important because chloroform is a toxic compound that can pose a health risk to humans and animals.
Regulations and Standards for Chloroform
The use, storage, and disposal of chloroform are regulated by a variety of laws and regulations. These regulations are designed to protect human health and the environment from the harmful effects of chloroform.The regulations governing chloroform include:* The Clean Air Act
- The Clean Water Act
- The Resource Conservation and Recovery Act
- The Toxic Substances Control Act
These regulations set limits on the levels of chloroform that can be released into the environment and require that chloroform be handled and disposed of properly.The enforcement of these regulations is the responsibility of the Environmental Protection Agency (EPA). The EPA works with state and local governments to ensure that chloroform is used, stored, and disposed of in a manner that protects human health and the environment.
Common Queries
What is the molecular structure of chloroform?
Chloroform, with the chemical formula CHCl3, has a tetrahedral molecular structure, consisting of a central carbon atom bonded to three chlorine atoms and one hydrogen atom.
What are the physical properties of chloroform?
Chloroform is a colorless liquid with a pungent odor. It has a boiling point of 61.2 °C, a melting point of -63.5 °C, and a density of 1.48 g/cm³.
What are the chemical properties of chloroform?
Chloroform is a reactive compound that can undergo a variety of chemical reactions, including substitution, addition, and elimination reactions.
What are the toxic effects of chloroform?
Chloroform is a toxic substance that can cause a range of adverse health effects, including central nervous system depression, respiratory depression, and liver damage.
What are the uses of chloroform?
Chloroform has been used historically as an anesthetic and as a solvent. Today, it is primarily used in the production of other chemicals.
